how to work out rate of reaction
Web In a first-order reaction the reaction rate is directly proportional to the concentration of one of the reactants. Write out the equation for calculating the rate of enzyme activity Rate Change Time In this case Rate Amount of product formed.
 |
| Solved Use The Data Provided And Calculate The Average Reaction Rate In Mol Ls For The Consumption Of Hydrochloric Acid Between 10s And 30s 3 Marks Time S Hci Mol L 1 000 10 |
Web Now we need to calculate the rate order with respect to the other reactant.
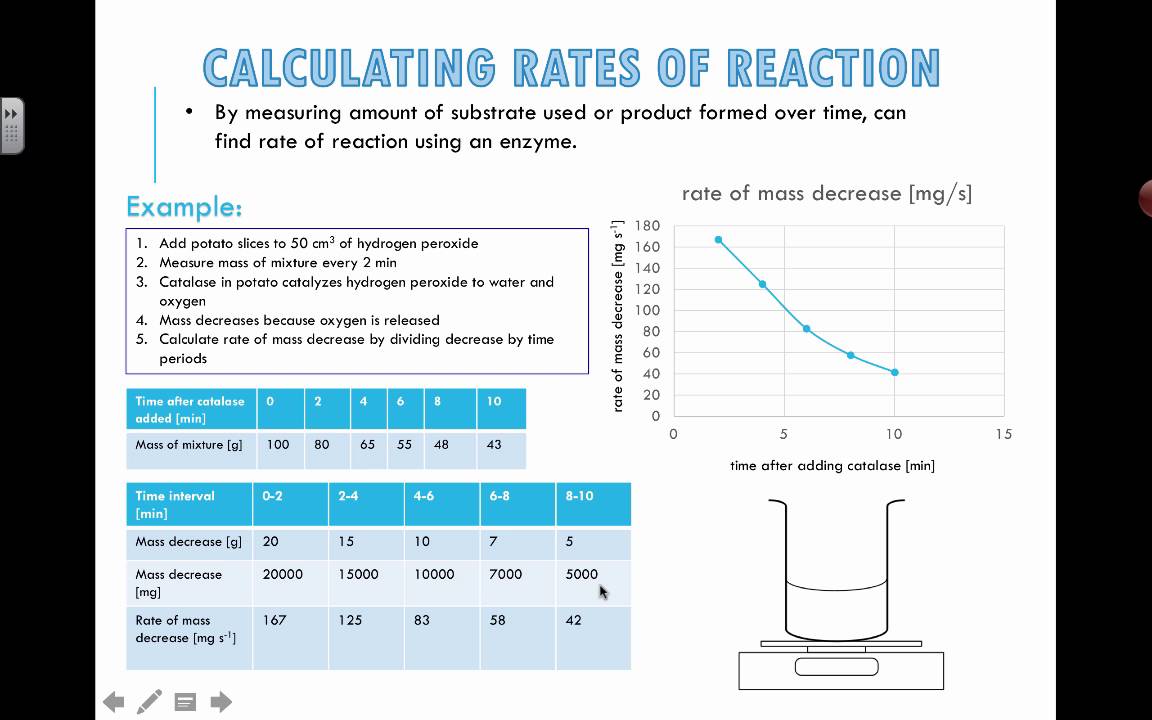
. The overall order of reaction. Web As mentioned earlier the rate of reaction can be calculated a couple of different ways. Web For a reaction of A B the reaction rate is change of concentrationtime. Web The reaction rate can depend on how concentrated our reactants are.
Assume we have a reaction 2A 3B. A chemical reactions rate law is an equation that describes the relationship between the. The initial rate of a reaction is the instantaneous rate at the start of the reaction ie when t 0. Web Again this is given by the change in concentration divided by the time taken - in other words the change in y-values divided by the change in x-values.
The method chosen usually depends on the reactants and products involved and how easy it is to measure changes. Web Measuring a rate of reaction There are several simple ways of measuring a reaction rate. The initial rate can be calculated using the negative slope of the curve at t0 of the reactant. The graph below shows the results of an enzyme rate.
Web The initial rate of a reaction is the instantaneous rate at the start of the reaction. I Rate of reaction frac14fracrmdleft. It can be measured as the rate of. First-order reactions often have the general form A products.
The initial rate is equal to the negative of the slope. AA bB cC dD the general. Web Here is how to find the gradient and therefore reaction rate. Decrease in concentration of A increase in concentration of B If.
Web There are different ways to determine the rate of a reaction. Web Calculate the rate of reaction. Web A reaction rate can be reported quite differently depending on which product or reagent selected to be monitored. Web Here we could write the equation for rate of reaction as r a t e Δ A Δ t Δ B Δ t Here the rate is expressed in two ways -In a way in which we have taken the.
Web This is a long answer. Web Calculate i the rate of reaction ii the rate of change of concentration of rmN_2rmO_5 Ans. Web Therefore the Rate of Reaction Δ A Δ t Δ B Δ t The above terms for the rate of disappearance of A and rate of appearance of B are average rates of reaction. You calculate the rate of reaction from the slope of a graph of concentration vs.
Web 44384 views Nov 21 2019 In this video I will teach you how to calculate the initial rate of reaction from a graph quickly and easily using the tangent method. The order of reaction with respect to reactant A is calculated as follows. First the general rate of reaction formula that involves the rate constant. For example if a gas was being given off during a reaction you could take some.
Pick two points on that tangent line. Web In these cases a tangent can be used to find the reaction rate at any one point on the graph. Draw a tangent to the curve of where you want to find that rate of reaction.
 |
| Rate Of Reaction Definition And Factors Affecting Reaction Rate |
 |
| 3 Ways To Determine Order Of Reaction Wikihow |
 |
| C2 4 Rates Of Reaction Secondary Science 4 All |
 |
| The Effect Of Temperature On Rates Of Reaction |
 |
| How Do You Calculate The Reaction Rate A Plus Topper Howtocalculaterateofreaction Reaction Rate Chemistry Classroom Chemistry Notes |
Posting Komentar untuk "how to work out rate of reaction"